Chempak Vapors Technical Information (Long)
|
© Copyright 1998 Madison Technical Software Inc. Used with permission.
Note: The information in this Appendix was taken from Section 8 of the Chempak Operating & Reference Manual, Version 4, Windows Edition, Issue: January 1998. Section numbering was left unchanged, and some sections that were judged not relevant were not included so the numbering is not sequential.
Note: Notation and references in this Appendix apply only to this Appendix, and are referenced at the end.
8.1 General
This section sets out the data sources, correlations and estimation methods used in the CHEMPAK database. In putting together the database, the methods and sources were selected in the following order of preference.
· Published experimental data
· Published correlations based on experimental data
· Specific category correlations
· General estimation methods
Madison Technical Software has followed the general recommendations in Reid and in Danner and Daubert as far as selection of specific category correlations and general estimation methods are concerned. In selecting specific compound data, a combination of sources has been used wherever possible. Important sources of specific compound data used by CHEMPAK are:
· Reid et al
· Perry et al
· J Chem Eng Data
· Daubert & Danner
· ESDU publications
· API Technical Data Book - Petroleum Refining
· International Critical Tables
· CRC Handbook
· Vargaftik
In many cases, the compound property values are a combination of published data, published correlations and general estimation methods. Several properties in certain compound categories have been estimated or adjusted by Madison Technical Software. It has been our policy to adopt and maintain a critical approach to available data sources and correlation methods.
The following sections set out details of the correlations and estimation methods used. In certain cases, the user is directed to the original references, particularly where the method is complex.
Data sources for Aqueous Solutions/Heat Transfer Liquids are published experimental data and correlations based on experimental data.
8.2 Physical Constants
8.2.1 Critical Temperature
The great majority of values are believed to be experimental. Where values had to be estimated, the Joback method was used.
8.2.2 Critical Pressure
Most of the values are experimental. In cases where experimental data were not available, the critical pressure was derived from the Joback method.
8.2.3 Critical Volume
A majority of the values are experimental. A great majority of the remaining compounds for which experimental values were not available had accurate boiling-point volumes available from which critical volume estimates were derived using the Tyn and Calus correlation. For a few substances, estimates of the critical volume were derived from the Joback method.
8.2.4 Normal Boiling Points
All values are believed to be experimental. In some cases, the values were slightly adjusted for vapor pressure.
8.2.6 Acentric Factors
The acentric factor is defined as
w = -log10(Pvr at Tr = 0.7) - 1
In all cases the acentric factor was derived from the vapor pressure correlation ( see section 8.8)
8.2.7 Joback Group Contribution Method
The Joback method is used to derive values of Tc, Pc, Vc and Tf where no experimental data or other predictive method was available.
Tc = Tb/(0.584 + 0.965 Sum(Dt) - Sum(Dt)2)
Pc = 1/(0.113 + 0.0032 na - Sum(Dp))2
Vc = 17.5 + Sum(Dv)
Tf = 122 + Sum(Df)
where na is the number of atoms in the molecule and the D contributions are given by Joback and by Reid et al (1987). Error magnitudes for the Joback method are as follows:
· Critical Temperature: average error about 1%
· Critical Pressure: average error about 5%
· Critical Volume: average error about 2%
· Freezing Point: average error about 11%
8.2.8 Tyn & Calus Relation
Tyn & Calus showed a close (< 3% error) relation between molar volume at normal boiling point and the critical molar volume of the form,
Vb = a Vcn
a = 0.285
n = 1.048
8.8 Vapor Pressure
The vapor pressure is expressed in its reduced form
Pvr = Pv/Pc
Reduced vapor pressure varies from very low values at freezing point to unity at the critical point.
8.8.1 Published Correlations
The experimental correlations are commonly given in the following formats:
Wagner Equation
ln(Pvr) = (aX + bX1.5 + cX3 + dX6)/Tr
with
X = 1 - Tr
FKT Equation
ln(Pv) = a + b/T + cln(T) + dPv /T2
Antoine Equation
ln(Pv) = a + b/(T + c)
8.8.2 Gomez-Thodos Vapor Pressure Equation
Gomez-Nieto and Thodos give the following equation:
ln(Pvr) = B(1/Trm - 1) + G(Tr7 - 1)
G = aH + bB
a = (1 - 1/Tbr)/(Tbr7 - 1)
b = (1 - 1/Tbrm)/(Tbr7 - 1)
H = Tbrln(Pc/Pb)/(1 - Tbr)
For non-polar compounds,
B = -4.267 - 221.79/(H2.5exp(0.038 H2.5)) + 3.8126/exp(2272.33/H3) + D
m = 0.78425 exp(0.089315 H) - 8.5217/exp(0.74826 H)
D = 0
except D = 0.41815 for He, 0.19904 for H2, 0.02319 for Ne
For polar non-hydrogen-bonding compounds (e.g. ammonia and acetic acid),
m = 0.466 Tc0.1667
G = 0.08594 exp(0.0007462 Tc)
B = (G - aH)/b
8.8.2 Gomez-Thodos Vapor Pressure Equations
For polar hydrogen-bonding compounds (water, alcohols),
m = 0.0052 M0.29Tc0.72
G = (2.464.M) exp(0.0000098 MTc)
B = (G - aH)/b
The advantages of this method are,
· fit guaranteed at T = Tb and T = Tc
· good performance with polar compounds
· good performance over Tr = 0.5 to 1
In addition, tests carried out by Madison Technical Software show the clear superiority of this method especially at low temperatures over the Lee-Kesler method.
8.8.3 Lee-Kesler Vapor Pressure Equation
Lee and Kesler give the following vapor pressure equation:
ln(Pvr) = f(0) + wf(1)
w = acentric factor
f(0) = 5.92714 - 6.09648/Tr - 1.28862 ln(Tr) + 0.169347 Tr6
f(1) = 15.2518 - 15.6875/Tr - 13.4721 ln(Tr) +0.43577 Tr6
The characteristics of this equation are,
· guaranteed fit at Tr = 1 and 0.7
· accurate for non-polar compounds
This equation is used in the Lee-Kesler and Wu & Stiel equations of state.
8.8.4 Interpolation and Extrapolation
In many cases an accurate empirical equation is known which does not extend to the critical point or to the freezing point. The approach taken here is to fit the Wagner equation by least squares to the empirical equation and use the Wagner equation to extrapolate to the freezing point and to the critical point.
Extrapolation by this method to the critical method is a very accurate procedure. Extrapolation to the freezing point is less accurate but it does provide reasonable values.
In CHEMPAK, the vapor pressure correlations set out in this section are used to provide the basic data. Empirical relations are used wherever possible.
8.9 Vapor Viscosity
The methods of Lucas are employed here. The equations take two forms:
· Low Pressure (< 2 atm)
· High Pressure (> 2 atm)
8.9.1 Low-Pressure Equation
v0 = a0k0{a1 + a2Trn + a3exp(a4Tr)+ +a5exp(a6Tr)}Fp0,Fq0
v0 = low pressure viscosity in kg/ms
k0 = M0.5Pc0.667Tc0.1667
with Pc in Pa and Tc in K
a0 = 0.0026373
a1 = 0.018
a2 = 0.807
a3 = -0.357
a4 = -0.449
a5 = 0.340
a6 = -4.058
n = 0.618
The low pressure polar correlation is given by,
Fp0 = 1 0 < mr < 0.022
Fp0 = 1 + f(Zc) 0.022 < mr < 0.075
Fp0 = 1 + f(Zc).g(Tr) 0.075 < mr
f(Zc) = 30.55(0.292 - Zc)1.72
g(Tr) = 0.96 + 0.1(Tr - 0.70)
mr = 52.46 m2Pc/Tc2
m = dipole moment, debye
Pc = critical pressure, bar
Tc = critical temperature, K
8.9.1 Low-Pressure Equation
In the above equations, if Zc > 0.292 the Fp0 is taken as unity.There are only a few compounds that are polar with Zc sufficiently larger than 0.292 to make Fp0 significantly different from unity. The evidence in any case is scanty.
The low-pressure correction for quantum gases (hydrogen, helium and deuterium), Fq0, is given by,
Fq0 = 1.22 Q0.15{1 - 0.00385(12 - Tr)2/M}
with
Tr < 12
Q = 1.38 for helium
0.76 for hydrogen
0.52 for deuterium
8.9.2 High-Pressure Equation
Define,
Z1 = v0/a0k0
For Tr <=1, we define,
Z2 = 0.60 + 0.76 Prm + (1 - Tr)(6.99Prn - 0.60)
m = 3.262 + 14.98 Pr5.508
n = 1.39 + 5.746 Pr
For Tr > 1 define,
Z2 = Z1{1 + aPre/(bPrf + 1/(1 + cPrd))}
with,
a = (a1/Tr)exp(a2Trn)
b = a(b1Tr - b2)
c = (c1/Tr)exp(c2Trm)
d = (d1/Tr)exp(d2Trp)
e = 1.3088
f = f1exp(f2Trq)
a1 = 0.001245
a2 = 5.1726
n = -0.3286
b1 = 1.6553
b2 = 1.2723
c1 = 0.4489
c2 = 3.0578
m = -37.7332
d1 = 1.7368
d2 = 2.2310
p = -7.6351
f1 = 0.9425
f2 = -0.1853
q = 0.4489
then,
Y = Z2/Z1
Fp = (1 + (Fp0 - 1)/Y3)/Fp0
Fq = (1 + (Fq0 - 1)(1/Y - 0.007(ln(Y))4))/Fq0
v = v0YFpFq
8.10 Vapor Conductivity
The method of Ely and Hanley is adopted for both high and low pressure vapor conductivity. The method is based on hydrocarbon data but gives reasonable values for non-polar non-hydrocarbons. Errors are usually less than 10% except for highly polar compounds. There are few experimental data and no satisfactory correlations for high pressure conductivity for polar compounds.
The vapor conductivity is given by,
k = k1 + k2 + k3
with,
k1 = 1944 v0H(1 + 0.042332(Cvo - 3R/2))
k2 = r0H(b1 + b2(b3 - ln(T0/b4))2)
k3 = k30k31(k32 + k33k34) - 1
k30 = H/1000
k31 = exp(a1 + a2/T0)
k32 = exp(a3 + a4/T01.5)r00.1
k33 = 6.1843(r0 - 1)r00.5
k34 = a5 + a6/T0 + a7/T02
The parameters in the above equations are given by:
v0 = low pressure methane viscosity = Sum(cnT0(n-4)/3)
c1 = 2.90774E-01
c2 = -3.31287E-01
c3 = 1.60810E-01
c4 = -4.33190E-02
c5 = 7.06248E-03
c6 = -7.11662E-04
c7 = 4.32517E-05
c8 = -1.44591E-06
c9 = 2.03712E-08
H = 16.04 f0.5/M h0.667
Cv0 = low pressure specific heat at constant volume in J/mol.K
R = gas constant = 8.314 J/mol.K
M = molecular weight g/mol
f = TcF1/190.4
h = VcF2/99.2
Tc = critical temperature, K
Vc = critical volume, cc/mol
F1 = 1 + (w - 0.011)(0.56553 - 0.86276 ln(T*) - 0.69852/T*)
F2 = {1 + (w - 0.011)(0.3949(V* - 1.02355) - 0.93281(V* - 0.75464)ln(T*)}(0.288/Zc)
w = acentric factor
Zc = critical compressibility
T* = Tr for Tr <= 2
= 2 otherwise
V* = V/Vc for 0.5 < V/Vc < 2
= 0.50 for V/Vc <= 0.50
= 2 otherwise
b1 = -2.5276E-04
b2 = 3.3433E-04
b3 = 1.1200E+00
b4 = 1.6800E+02
a1 = -7.1977E+00
a2 = 8.5678E+01
a3 = 1.2472E+01
a4 = -9.8463E+02
a5 = 3.5947E-01
a6 = 6.9798E+01
a7 = -8.7288E+02
r0 = 16.04 h/V
T0 = T/f
V = specific volume, cc/mol
T = temperature, K
8.11 Ideal Gas Thermodynamic Properties
Ideal gas thermodynamic properties (i.e. at low pressure) are required for the estimation of the thermodynamic properties of liquids and gases.
8.11.1 Method of Joback
For those substances not covered by published correlations, the group contribution method of Joback is used to estimate Cp0 and the heats of formation.
Cp0 = a + bT + cT2 + dT3
with,
a = Sum(njDa) - 37.93
b = Sum(njDb) + 0.210
c = Sum(njDc) - 3.91E-04
d = Sum(njDd) + 2.06E-07
where nj is the number of groups of type j and the D contributions are for this type of group. T is in deg K. The user is referred to Joback for details. Reid et al (1987) gives values of the group contributions. The error associated with this method is usually less than 3%.
8.11.2 Low-temperature values of Cp0
To our knowledge, no general estimation method exists for Cp0 below about 260 K. In addition, empirical datapoints and correlations in this region are not generally available. In order to extend the applicability of the estimation methods to temperatures below 260 K, extrapolation of known values and correlations was investigated by Madison Technical Software.
Extrapolation as far as 50 K (-223 C) using monotonic power functions fitted to known values at higher temperatures gave estimates whose error did not exceed 10% at 50 K and which averaged about 2% in the range 100 K to 273 K. This extrapolation was tested against known values for over 80 hydrocarbons and non-hydrocarbons. Thermodynamic properties calculated are limited in CHEMPAK to temperatures above minus 100 C.
8.11.3 Derived Ideal Gas Properties
Ideal gas enthalpy and entropy are derived by integration of the ideal gas specific heat:
The ideal gas enthalpy, h0, is given by the integral of Cp0 from T0 to T
h0(T) = [C0T + C1T2/2 + C2T3/3 + C3T4/4]
evaluated between T0 and T.
The ideal gas entropy is given by the integral of Cp0/T from T0 to T
s0(T) = [C0lnT + C1T + C2T2/2 + C3T3/3]
evaluated between T0 and T. T0 is a zero-value reference temperature. The specific heat at constant volume is simply related to the specific heat at constant pressure:
Cv0 = Cp0 - R
8.12 The Equations Of State
8.12.1 General
The two equations of state employed in CHEMPAK are as follows:
1. The Lee-Kesler equation of state for non-polar compounds with moderate values of acentric factor
2. The Wu & Stiel equation of state for polar compounds or those with extreme values of acentric factor
The equations of state are used to predict the following vapor properties:
· Specific Volume
· Compressibility
· Expansion Coefficient
· Specific Heats
· Enthalpy/Internal Energy
· Entropy
· Heat of Vaporization
8.12.2 The Lee-Kesler Equation of State
The Lee-Kesler equation of state is a three-parameter (Tc, Pc and w) equation explicit in pressure with general applicability to compounds with low polarity and moderate acentric factors. The Lee-Kesler equation defines two fluids as follows,
· a simple fluid with zero acentric factor
· a reference fluid with acentric factor = 0.3978
The specific volume and other properties for any fluid are determined by interpolation between the simple fluid properties and the reference fluid properties using acentric factor as the interpolating variable. The properties of the simple and reference fluids are determined by a pair of PVT equations with identical form but with different parameters.
We define,
Pr = P/Pc
Tr = T/Tc
Vr = PcV/RTc
where P, Pc, T and Tc are the actual and critical pressures and the actual and critical temperatures of the fluid of interest.
First, Vr(0) is calculated using the constants for the simple fluid. Then Vr(r) is calculated using the constants for the reference fluid. From these quantities, Z(0) and Z(r) are calculated:
Z(0) = PrVr(0)/Tr Z(r) = PrVr(r)/Tr
The compressibility of the fluid of interest is calculated using,
Z = A(0)Z(0) + A(r)Z(r)
A(0) = 1-w/w(r)
A(r) = w/w(r)
w = acentric factor for fluid of interest
w(r) = acentric factor for reference fluid (0.3978)
The expansion coefficient, specific heat departures, enthalpy and, entropy departures are derived from the simple and reference PVT equations using the relations detailed in Lee and Kesler and in Reid and elsewhere. The component simple fluid and reference fluid values are combined by using relations of the same form shown above for compressibility. Danner & Daubert give typical maximum error values 20 kJ/kg for vapor enthalpy and 70 kJ/kg for liquid enthalpy. Typical errors for specific volume of vapors are 1 to 2%.
8.12.3 The Wu & Stiel Equation of State
The Wu & Stiel equation of state is a four-parameter equation of state developed as an extension to the Lee-Kesler equation of state to cover polar compounds and compounds with high values of acentric factor. The parameters of this equation of state are,
· Critical Temperature, Tc
· Critical Pressure, Pc
· Acentric Factor, w
· Polarity Factor, Y
The Wu and Stiel equation of state uses three reference fluids as follows:
· simple fluid with zero acentric factor
· reference fluid with acentric factor = 0.3978
· polar fluid (water) with acentric factor = 0.344 and polarity factor = 1.0
The specific volume and other properties for any fluid of interest are determined by interpolation between the properties of the reference fluids using acentric factor and polarity factor as interpolating variables.
The properties of the simple and reference fluids are determined from the Lee-Kesler equation of state. The properties of the polar fluid are determined from the Keenan equation of state for water. With Pr and Tr for the fluid of interest,
Z(0) = PrVr(0)/Tr from simple fluid equation
Z(r) = PrVr(r)/Tr from reference fluid equation
Z(p) = PrVr(p)/Tr from polar fluid equation
Z = A(0)Z(0) + A(r)Z(r) + A(p)Z(p)
A(0) = (1 - Y) - (w/w(r) - Yw(p)/w(r))
A(r) = w/w(r) - Yw(p)/w(r)
A(p) = Y
A(0) + A(r) + A(p) = 1
When Y = 0 these equations reduce to the non-polar Lee-Kesler formula. When Y = 1 and w = w(p), they reduce to the Keenan equation of state for water.
The expansion coefficient, specific heat departures, enthalpy, internal energy and entropy departures are derived from the simple, reference and polar PVT equations. The component simple fluid, reference fluid and polar fluid values are combined using relations of the same form as shown above in the case of compressibility. The polarity factor is determined from empirical density data in accordance with the relations set out by Wu and Stiel. Wu & Stiel report excellent results using this equation of state for polar compounds. Errors in specific volume were less than 1 to 2% for example for the vapor phase.
8.12.4 Calculation of Saturation Values
Fluid properties along the saturation line are solved for as follows. For a given value of Tr,
1. The simple fluid component properties are solved at Tr , Psr(O) where this latter quantity is the Lee-Kesler reduced saturation pressure at Tr and w = 0
2. The reference fluid component properties are solved at Tr and Psr(r) where this latter quantity is the Lee-Kesler reduced saturation pressure at Tr and w = 0.3978
3. The polar fluid component properties are solved at Tr and Psr(p) which is the reduced water saturation pressure at Tr with Psr(p) = Ps(p)/ Pc(p)
The compressibility of the fluid of interest is found by,
Zs = A(0)Zs(0) + A(r)Zs(r) + A(p)Zs(p)
This relation gives the saturation state of the fluid of interest by interpolation between the saturation states of the simple, reference and polar fluids at Tr rather than by interpolation between the three fluids at Tr and Psr where this latter quantity is the reduced vapor pressure of the fluid of interest.
The above relations are presented for the case of the Wu & Stiel equation of state. The relations for the Lee-Kesler equation of state may be obtained by setting the polar contribution equal to zero.
8.12.5 Enthalpy and Entropy Scales
All enthalpy and entropy values are quoted relative to a reference zero-value temperature T0. This temperature is taken in all cases to be the higher of 273.15 K and the normal melting point.
Two cases can be distinguished:
1. If the critical temperature is greater than or equal to 273.15 K then the enthalpy and entropy of the saturated liquid are taken to be zero at T0. In this case the enthalpy and entropy functions are,
h(T,P) - hLs(T0) = [h(T,P) - hv(T,P0)]es + [h0(T0,T)]ig - [hLs(T0) - hv(T0,P0)]es
s(T,P) - sLs(T0) = [s(T,P) - sv(T,P0)]es + [s0(T0,T)]ig - [sLs(T0) - sv(T0,P0)]es
2. If the critical temperature is less than 273.15 then the enthalpy and entropy of the low-pressure vapor are taken to be zero at T0. In this case the enthalpy and entropy functions are,
h(T,P) - hv(T0,P0) = [h(T,P) - hv(T,P0)]es + [h0(T0,T)]ig
s(T,P) - sv(T0,P0) = [s(T,P) - sv(T,P0)]es + [s0(T0,T)]ig
The zero point for water is taken to be 273.16 K in accordance with the Keenan equation of state for water. Liquid enthalpy and entropy are zero at that temperature and saturation conditions.
8.13 Properties Of Mixtures
8.13.1 Scope of Correlations
CHEMPAK provides the user with the facility of defining mixtures of compounds in the database which then may be stored in the database for future use. The properties of mixtures are calculated using the techniques set out in this section. The user should note the following:
A mixture can be formulated using any components in the database except other mixtures, aqueous solutions, heat transfer liquids and User-Defined Liquids.
No check is carried out by CHEMPAK on the chemical stability, compatibility or miscibility of the selected components or the defined mixture. The user must be satisfied as to the physical reasonableness of the formulation of the mixture.
The accuracy of the calculated properties can be expected to be better when the selected components are chemically similar to each other. The accuracy of the calculated properties of very dissimilar components is not known but may only be order-of-magnitude in some cases. It is to be expected that the errors involved with mixtures of non-polar compounds will be less than with mixtures of polar compounds.
The range of applicability of the correlations is as follows:
A low-limit temperature is defined corresponding to the maximum of the reduced melting points of the mixture components. Properties cannot be accessed at temperatures lower than this. A high-limit temperature of 1300 K or the pseudocritical temperature (whichever is the greater) is defined for vapor properties. Vapor properties cannot be accessed at temperatures greater than this. The high limit temperature for liquid mixture properties is the pseudocritical temperature of the mixture as defined by the Lee-Kesler rules. Liquid properties cannot be accessed at temperatures higher than this.
8.13.2 Mixture Critical Properties
Critical Temperature
Tc = Sum(xiVciTci)/Sum(xiVci)
where xi, Vci and Tci are the mole fraction, critical volume and critical temperature of component i. The reference used here is Li. This method is generally recommended by Reid and by Danner and Daubert. Deviations for binary hydrocarbon mixtures can be expected to be less than 4 K. Errors for multicomponent hydrocarbon mixtures average about 11 K. Errors for binary mixtures of non-hydrocarbons would be of this order.
Critical Volume
Vc = Sum(aiVci) + Sum(aiajbij) (all i,j)
ai = xiVci2/3/Sum(xiVci)
bii = 0 bij = 2cij(Vci + Vcj)
cij = Aij + Bijdij + Cijdij+2 +Dijdij3 +Eijdij4
dij = ABS{(Vci2/3 - Vcj2/3)/(Vci2/3 + Vcj2/3)}
The binary constants Aij thru Eij are given by,
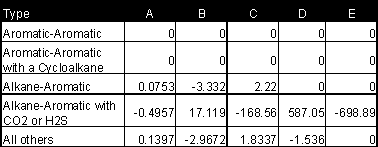
The reference for this method is Schick and Prausnitz. An average error of 10% can be expected for binary mixtures.
Critical Pressure
wm = Sum(xiwi)
Tcm = Sum(ZiTci)
Zi = xiVci/Sum(xiVci)
Tcm* = Sum(xiTci)
Pcm* = Sum(xiPci)
Pc = Pcm*[1 + (5.808 + 4.93 wm)(Tcm/Tcm* - 1)]
This method is based on a simplification of the method of Kreglenski and Kay as amended by Spencer, Daubert and Danner. Errors in the application of this method average about one bar - see Reid et al for details. This method is not applied when the mixture contains inorganic components. In this case the critical pressure is taken equal to the Lee-Kesler pseudocritical pressure.
Acentric Factor
In accordance with the Lee-Kesler rules,
w = Sum(xiwi)
wi = component acentric factor
xi = component mole fraction
8.13.8 Vapor Pressure
Accurate determination of the vapor pressures of mixtures normally requires a full vapor-liquid equilibrium model with knowledge of interaction parameters. Such a VLE model is not a part of CHEMPAK. In CHEMPAK, however, an approximation is used in order to be able to determine the mixture phase for given input T and P. The Lee-Kesler mixing rules give for the acentric factor,
wm = Sum(xiwi)
wi = -log(Pvri at Tr = 0.7) - 1
wm = -log(Pvrm at Tr = 0.7) - 1 = Sum(-xilog(Pvri at Tr = 0.7) - xi)
hence,
log(Pvrm at Tr = 0.7) = Sum(xilog(Pvri at Tri = 0.7))
The vapor pressure is represented for all compounds in the CHEMPAK database by the Wagner equation:
ln(Pri) = (aiXi + biXi1.5 + ciXi3 + diXi6)/Tri
Xi = 1 - Tri
Hence, for the mixture, the Wagner coefficients are given by,
am = Sum(xiai) bm = Sum(xibi)
cm = Sum(xici) dm = Sum(xidi)
where xi is the component mole fraction.
8.13.9 Thermodynamic Properties of Mixtures
The Lee-Kesler and Wu & Stiel equations of state are used to calculate the thermodynamic properties of liquid and vapor phases for all pure compounds in the database. To calculate the thermodynamic properties of mixtures, a set of pseudocritical constants are defined for each mixture and the properties are calculated in the usual way.
CHEMPAK uses the original Lee-Kesler rules as follows:
Zci = 0.2905 - 0.085 wi
Vci = ZciR0Tci/Pci
Vcm = Sum(xixj(Vci1/3 + Vcj1/3)3)/8
Tcm = Sum(xixj(Vci1/3 + Vcj1/3)3(TciTcj)1/2) / (8Vcm)
wm = Sum(xiwi)
Mm = Sum(xiMi)
Zcm = 0.2905 - 0.085 wm
Pcm = R0ZcmTcm/Vcm
R0 = Gas Constant
The Stiel polarity factor for a polar mixture is computed from the mixture liquid specific volume
8.13a Thermodynamic And Transport Properties Of Mixtures
8.13.10 Vapor Viscosity
The pure component equations are used with the following pseudocritical constants defined for each mixture per the formulation of Prausnitz and Gunn:
Tcm = Sum(xiTci)
Zcm = Sum(xiZci)
Vcm = Sum(xiVci)
Mm = Sum(xiMi)
Pcm = ZcmR0Tcm/MmVcm
8.13.11 Vapor Conductivity
The pure component equations are used with the following pseudocritical constants from Yorizane et al defined for the mixture:
Tcm = Sum(xixj(Vci1/3 + Vcj1/3)3(TciTcj)1/2)/(8Vcm)
Vcm = Sum(xixj(Vci1/3 + Vcj1/3)3)
wm = Sum(xiwi)
Zcm = 0.2905 - 0.085 wm
Pcm = ZcmR0Tcm/MmVcm
Mm = Sum(xiMi)
8.13.12 Ideal-Gas Mixture Properties
Ideal-gas mixture properties are taken as the mole fraction of the pure component properties. The pure component properties are defined per mole of substance.
8.14 Notation
C Specific Heat
e Expansion Coefficient
h Enthalpy
log Logarithm to base 10
ln Natural Logarithm
m Dipole Moment
M Molecular Weight
P Pressure
R Gas Constant
r Riedel Parameter
s Entropy
T Temperature
v Viscosity
V Specific Volume
w Acentric Factor
x Mole Fraction
Y Wu & Stiel Polarity Factor
Z Compressibility
Subscripts
b Boiling
c Critical
es Equation of State
f Freezing
ig Ideal Gas
L Liquid
m Mixture
0 Low Pressure
p Constant Pressure
ra Rackett
ref Reference
r Reduced
s Saturated
v Vapor
v Constant Volume
Superscripts
(o) Simple Fluid
(r) Reference Fluid
(p) Polar Fluid
8.15 References
API Technical Data Book - Petroleum Refining, 4 Vols, API, Washington DC, 1988
CRC Handbook of Chemistry and Physics, CRC Press Boca Raton 1991
Danner R P and Daubert T E , Data Prediction Manual, Design Institute for Physical Property Data , AIChE, NY 1983
Daubert T E and Danner R P, Physical and Thermodynamic Properties of Pure Chemicals, Data Compilation, AIChE, Hemisphere NY 1989
Engineering Sciences Data Units (ESDU), (9 Vols Data Compilation), London, England
Gomez-Nieto M and Thodos G, Ind Eng Chem Fundam, Vol 17, 45, 1978, Can J Chem Eng, Vol 55, 445, 1977, Ind Eng Chem Fundam, Vol 16, 254, 1977
Hankinson and Thomson, AIChEJ, Vol 25, 653, 1979
International Critical Tables, National Research Council, 7 Vols, McGraw-Hill, NY 1926
Joback K G, SM Thesis, MIT, June 1984
Keenan et al, Steam Tables, John Wiley, NY 1978
Knapp et al, Chem Data Ser, Vol VI, DECHEMA 1982
Kreglewski and Kay, J Phys Chem, Vol 73, 3359, 1969
Letsou A and Stiel L I, AIChEJ, Vol 19, 409, 1973
Le Bas G, Molecular Volumes of Liquid Chemical Compounds, Longmans Green, NY 1915
Lee B I and Kesler M G, AIChEJ, Vol 21, 510, 1975
Li C C, AIChEJ, Vol 22, 927, 1976
Lucas K, Chem Ing Tech, Vol 53, 959, 1981
Missenard F A, Rev Gen Thermodyn, Vol 101, 649, 1970
Morris P S, MS Thesis, Polytech Inst Brooklyn, NY 1964
Perry et al , Chemical Engineer's Handbook (various editions), McGraw Hill, NY
Plocker U J et al, Ind Eng Chem Proc Des Dev, Vol 17, 324, 1978
Prausnitz and Gunn, AIChEJ, Vol 4, 430 and 494, 1958
Reid R C at al, Properties of Liquids and Gases, 3rd Ed, McGraw Hill, NY 1977, 4th Ed, McGraw Hill, NY 1987
Schick and Prausnitz, AIChEJ, Vol 14, 673, 1968
Spencer and Danner, J Chem Eng Data, Vol 17, 236, 1972
Spencer, Daubert and Danner, AIChEJ, Vol 19, 522, 1973
Stiel and Thodos, AIChEJ, Vol 8, 229, 1962
Teja A S at al, Chem Eng Sci, Vol 33, 609, 1978, AIChEJ, Vol 26, 337 & 341, 1980, Chem Eng Sci, Vol 36, 7, 1981, Ind Eng Chem Fundam, Vol 20, 77, 1981, Chem Eng Sci, Vol 37, 790, 1982, J Chem Eng Data, Vol 28, 83, 1983, Ind Eng Chem Proc Des Dev, Vol 22, 672, 1983
Thomson, Brobst, Hankinson, AIChEJ, Vol 28, 671, 1982
Tyn M T and Calus W F, Processing, Vol21(4), 16, 1975
Van Velzen D et al, Ind Eng Chem Fundam, Vol 11, 20, 1972
Van Velzen et al, Liquid Viscosity etc, Euratom 4735e, Joint Nuclear Research Centre, ISPRA Establishment, Italy 1972
Vargaftik N B, Tables on Therm Props Liq & Gases, 2nd Ed, Hemisphere, Washington DC, 1975
Wu G Z A and Stiel L I, AIChEJ, Vol 31, 1632, 1985
Yorizane et al, Ind Eng Chem Fundam, Vol 22, 454, 1983