Network Implementation
By applying the Continuity Equation at a Branch about junction i and branches j, we get
|
|
(1) |
By applying the Energy Equation at a Branch about junction i and branches j, we get
|
|
(2) |
To obtain a valid solution in a network, the stagnation pressure at each branch point will be equal and the mass flow and energy flow will sum to zero at the branch. Although the stagnation pressures at the pipe endpoints will equal that at the connecting junction, this is not true for enthalpy (and temperature). The enthalpy at the end of a pipe will be that enthalpy that results from the heat transfer in the pipe. Once the fluid flows into the junction, the enthalpy will change to the mixture enthalpy, or the average enthalpy of all inflowing pipes.
If only one pipe flows into the branch, the branch enthalpy will equal the enthalpy coming out of the pipe. But if more than one pipe flows into the branch, the enthalpy will reach an averaged condition. It is this averaged enthalpy that results from mixing of two or more streams at different enthalpies. This enthalpy is supplied to all outflowing pipes. The preceding discussion in depicted in Figure 1 and follows equation 3.
|
|
(3) |
where the + superscript indicates the summation applies only to positive inflows into the junction.
To provide converged solutions in the shortest time possible, matrix solution techniques are required. Matrix techniques allow all pipes to be solved together, which offers speed and the best convergence characteristics.
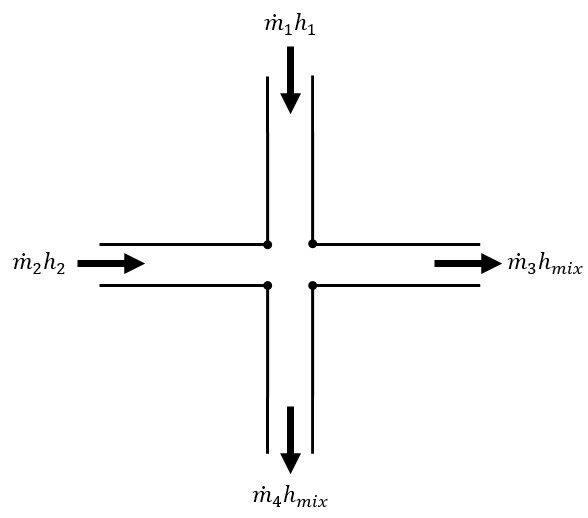
Figure 1: Flow weighted mixing and energy balance
Energy Balances with AFT Standard fluids
The AFT Standard fluids do not have enthalpy data, so Fathom uses the Reference Enthalpy Method, described in the following equation, to calculate enthalpy:
Therefore Equation 3 can be expressed as the following:
|
|
(4) |
The reference enthalpy and temperature is entered in the fluid library, and should be for a state that is as near as possible to the operating conditions of your model. As seen from the equation, the pressure dependence of enthalpy is ignored. In many cases, this assumption is acceptable.
Chempak and NIST REFPROP fluids have enthalpy data and Equation 3 can be used directly.



